Lesley Hymers, Mining Matters
Observe

Have you ever been on a hike and seen a rock outcrop? Have you seen rocks on a beach? Then, you may have seen minerals and crystals with your own eyes!
You can even see minerals and crystals at the grocery or drugstore AND in your kitchen! Minerals and crystals are visible all around us in nature and in our human-made environment.
Now what happens when you add salt to a pot of boiling water, Epsom salts to your bath water or Borax to a load of laundry? These crystals look like they disappear, blending in with the water. They’re actually dissolving!
Can you reverse this process? Do you think you can create crystals?
Research
 What are minerals and crystals?
What are minerals and crystals?
Are they solid, liquid or gas? Minerals and crystals are actually naturally occurring solids made of the same thing throughout.
Do minerals and crystals occur in different shapes?
If so, what are the shapes and how many of them are there?
Let’s take a look at crystals first! When we look at how crystals form, their shapes, how they behave and how they are classified, it’s called crystallography. What makes a crystal a crystal is that the particles, or more specifically, the atoms, are arranged a repeating pattern throughout. This arrangement influences how crystals look.
There are seven arrangements crystal atoms can take: cubic, tetragonal, triclinic, orthorhombic, hexagonal, monoclinic, and trigonal.
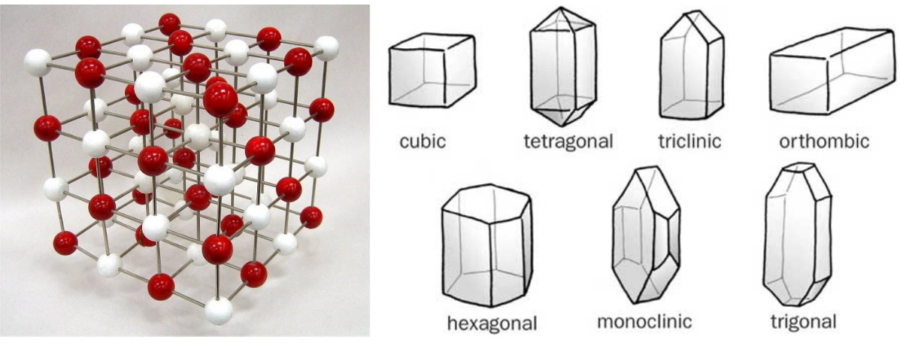

Minerals are a type of crystal that are found in nature, made up of the same substance throughout. They tend to form into the same shapes when they have time and space to grow. When we study minerals, such as how how they are formed, where on Earth they are found, what they are made of, their different properties, and how they are named, or classified, it’s a type of Earth Science called mineralogy. Crystallography is actually a branch of mineralogy.
How fast do crystals grow?
How fast crystals grow depends on many factors, including the temperature of the liquid they’re dissolved in, such as water, how much of the mineral is dissolved, and the humidity. But it all starts with what Earth scientists call a nucleation point: the first building block that gets the ball rolling. This could be anything from a surface crack or rough patch or a crystal that is already there.
How do crystals form?
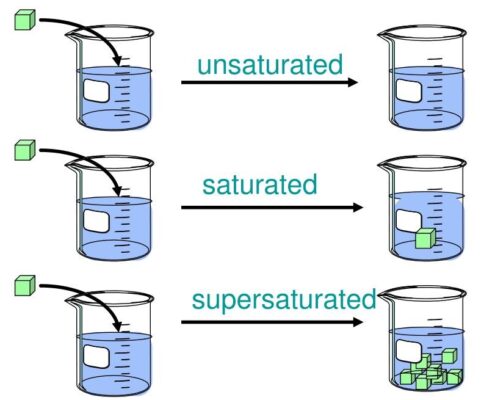
Crystals can form from liquid mixtures called solutions. Solutions are made up of solutes, the minor portion that is dissolved in the solution, and the solvent, which is the major component that dissolves the minor portion, such as water. There are three types of solutions: unsaturated, saturated and supersaturated. An unsaturated solution contains a small fraction of solutes. In the diagram to the right in the unsaturated solution, we see that the solute (salt) dissolves completely in the solvent (water). A saturated solution is when the solvent dissolves as much solute as it can. In some situations, a solution may become supersaturated where it can hold more dissolved substance than should be possible. These solutions aren’t stable and will return to a saturated state with minor disruptions, like changes in temperature.
Different degrees of order in crystal and non-crystal structures
The rate at which a crystal grows will affect the resulting crystal. A crystal that grows quickly will likely have an irregular pattern, whereas while a crystal that grows slowly will likely have have a more consistent arrangement. The size of the crystals grown from a solution depends on the number of nucleation points. The more nucleation points there are, the smaller the crystals will likely be and the fewer the nucleation points, the larger crystals will likely be!
What other questions about crystals and minerals do you have? Write them down and find the answers!
Want to learn more about minerals and crystals?
You can learn more about mineralogy and crystallography by doing more research through Mining Matters’ Creating Mineral Mates activity or visit the University of Waterloo Earth Science Museum and the International Gem Society website to research minerals and crystals.
Stay tuned for the activity to get us to think like an Earth Scientist!

 What are minerals and crystals?
What are minerals and crystals?