Can you fish for ice? Place some ice cubes in a cup of water and try to grab an ice cube with your piece of string! We run this staple activity every year at Science Rendezvous. Since we weren’t able to to bring this activity to you in-person this year, we bring it to you virtually!
Did you catch an ice cube? It’s a bit challenging without some salt. How do you think we can use salt to help fish for ice? Brainstorm some ideas and try some out!

Now that you’ve done some exploring, did you figure out the trick? Our trick is to place the string across the ice cubes, sprinkle some salt onto the ice cubes and string, and then wait. After a minute or so, pull out the string, and the ice should come with it!
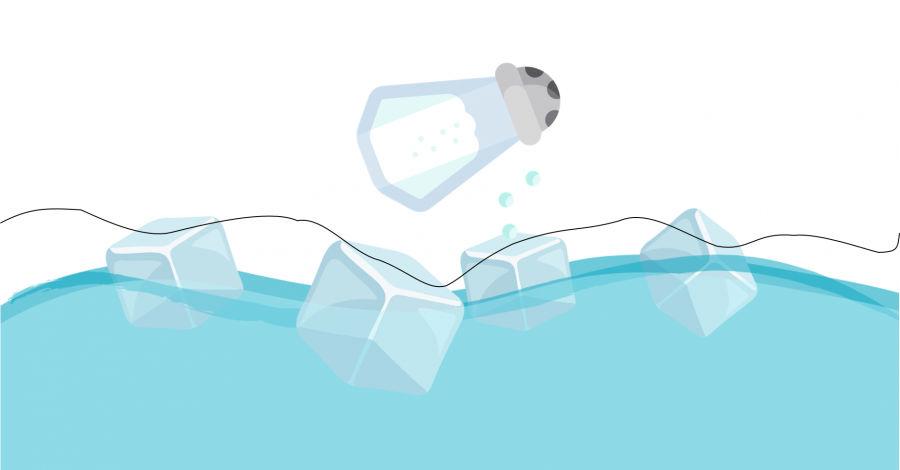
Why does that work?
Have you ever lived in or visited a snowy or icy place? Have you ever seen salt being spread over the roads, stairs and walkways after it snows or when they get icy? The reason we do this is the same reason we can fish for ice cubes using salt!
All matter, which is any and everything that takes up space and has weight, is made up of particles. Solid particles are usually tightly packed together, vibrating and held together by attractive forces. Liquid particles are more free-flowing and interact with each other more loosely than solid particles. But water is a unique type of matter. With water, the solid particles like to form crystal patterns in its icy state, making the particles further apart than when the liquid particles are free-flowing. Since ice is then less tightly packed, or dense, than water, ice will float on water! This is an important characteristic of water that allows our earth to have glaciers and oceans of marine animals underneath sheets of ice.
Changing phases
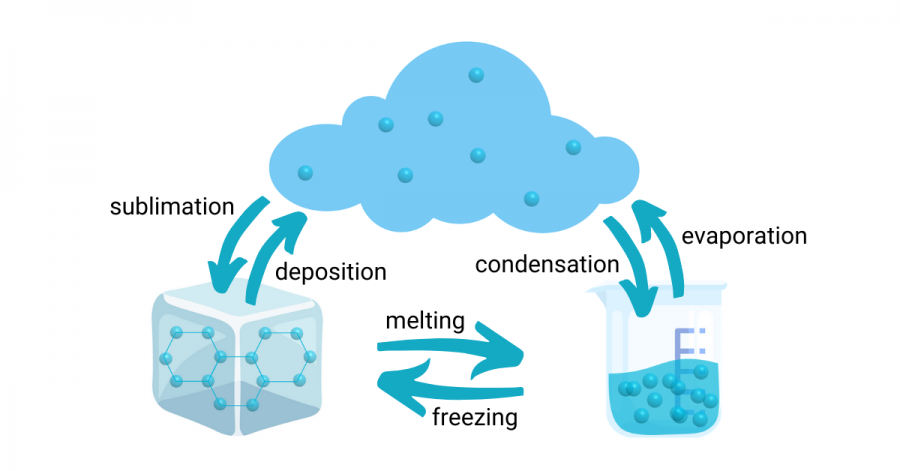
When things melt, they go from solid to liquid. The particles start to slow down as the temperature gets colder, making liquid turn into solid. For water, 0 degrees C is when it becomes ice. But when you add salt, water will freeze at a colder temperature and ice will melt more easily.
Why is that? Well, salt has a great relationship with water! It dissolves quite readily in liquid water because of the attractive forces between salt and water. Its presence on the ice floating in water speeds up the melting and disrupts the ice crystal structures. If you add enough salt to your water, the saltwater could freeze at -6 degrees Celsius instead of 0 degrees Celsius. You can also see this happening with seawater, which freezes at around -2 degrees Celsius.
But in our experiment, there was only a small amount of salt so the water is able to refreeze around the ice cube and string, trapping the string to the ice. Now, you can pull the ice cube out with your string with the same science we use to make ice cream!