
Get ready to INNOVATE with Science Rendezvous, returning May 11, 2024!
Science Rendezvous returns on May 11, with a new lineup of exciting events, activities, and demos. Stay tuned for more information about our event.
You can also follow us on X (formerly Twitter) @SciRenMUN for more details!


Photo credit: www.kiplinger.com
Someone is stealing the pets in your neighbourhood!! You have been called in as Chief of the PPO – People’s Pet Operative – to find the culprit. You have figured out where the animals are being hidden but you need a special key to access the building. In order to make sure you have the right key, you need to complete a Science Chase at Memorial University – each question you answer correctly earns you a key but you don’t know if it is the right one so you need to solve them all! The pressure is on to save the animals!
Are you able to complete this challenge and return the pets safely to your neighbors? If you’re up to the task, start your adventure in the Physics department!
This Site's Science Chase Resources:
Investigating Eddy Currents

Your first stop in the quest to rescue the pets is with Dr. Mike Morrow in the Physics department.
He makes things float in air (levitate) – watch the video below to see how and collect your first key!
As the magnet falls inside the tube, the field inside a section of the aluminum tube changes as the magnet approaches and then changes in the other way as the magnet falls past that section of tube. This causes currents (eddy currents) to circulate around the aluminum tube. From here, you can think of the slowing of the magnet in terms of energy (the energy to drive the current “uses up” some of the energy of the falling magnet and slows it down) or in terms of force (the “induced” current circulating below the falling magnet creates a magnetic field that pushes up on the magnet and the “induced” current circulating above the falling magnet creates a magnetic field that pulls up on the falling magnet.
Practice Question #1:
True of False: A magnet will fall faster inside an aluminum (conducting, non-magnetic) tube than along the outside of the tube?
Medical School 1 - BodyWorks
Congratulations on getting your first key! You can’t test it until you collect all of the keys to see if you have the right one.
The next 4 challenges are at the Faculty of Medicine and show you some of the important work that is done in hospitals in order to diagnose illnesses and disease.
Making discoveries by medical research is hard but it is very important for teaching new medical students.
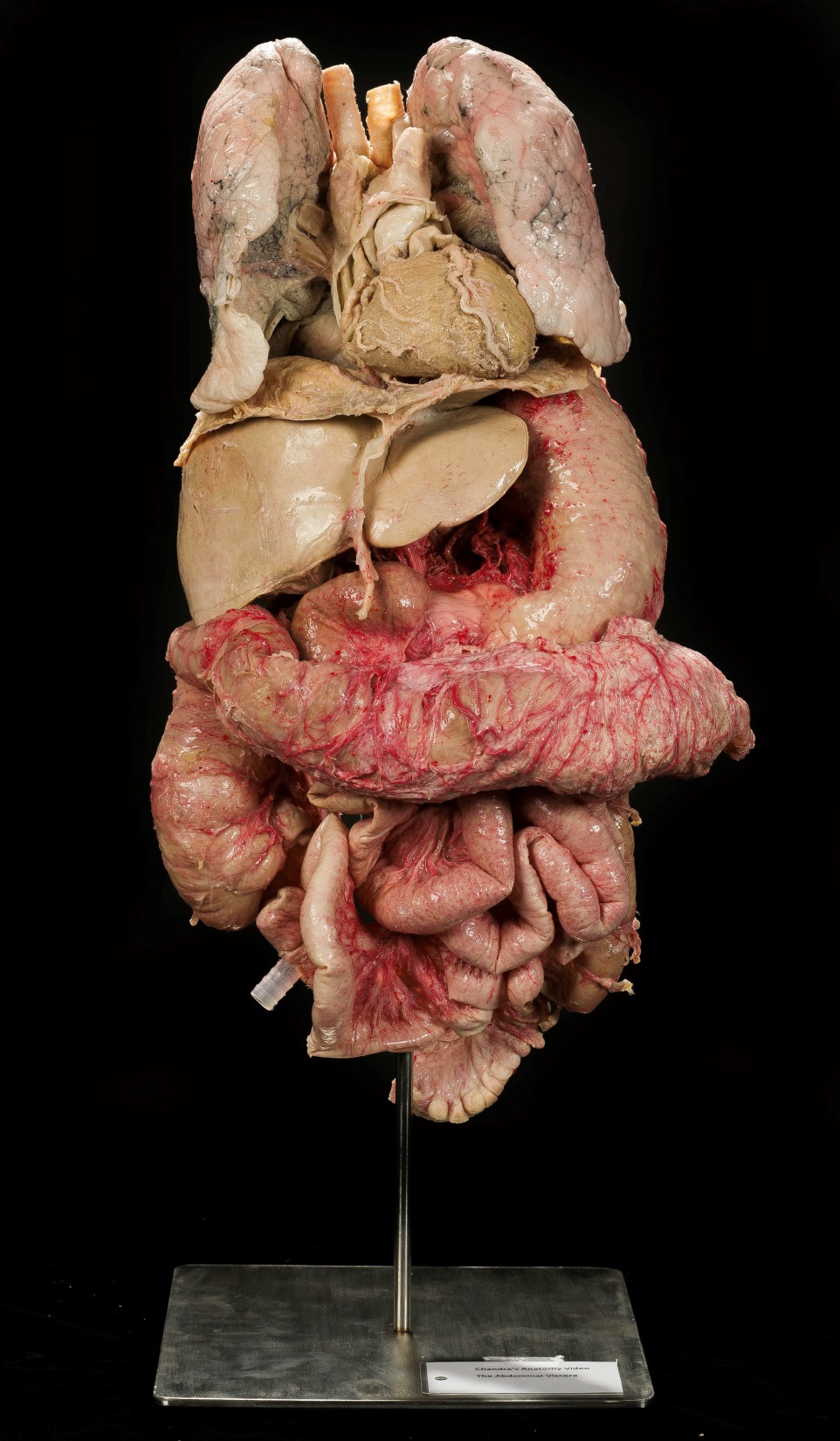
In the first video, Dr. Patricia Cousins explains how the medical school does that uses real bodies to contribute to science and research.
Practice Question #2:
Why are sample donations infused (injected with) rubber?
Medical School 2 - Discovering Cells
You’re on a roll with 2 keys! Well-done!
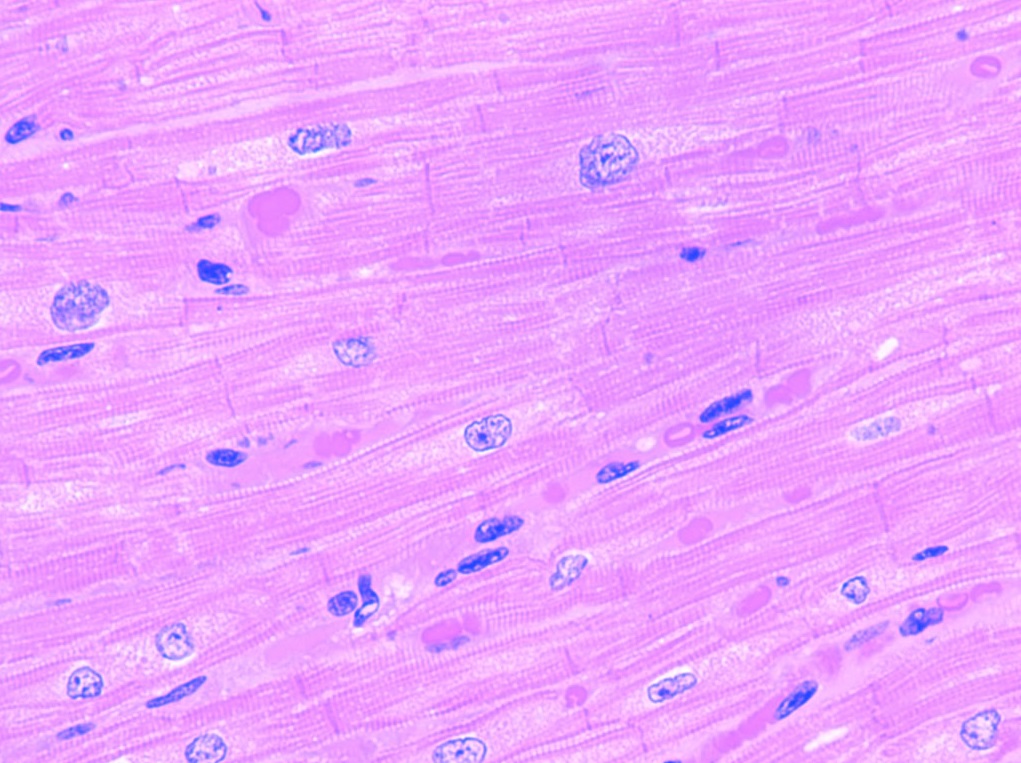
Have you ever wondered what your cells look like on the inside? The parts inside of your cells (organelles) have a lot of important jobs to do every day. Watch Danielle Gardiner in the second video to see all the important organelles at work each day, even when you sleep!
Practice Question #3:
What does histology do?
Medical School 3 - Investigating the Electron Microscope

And just like that you are at your third stop in the Medical School! You are doing great!
Since cells are so small, how do scientists and doctors see inside? If you guessed a microscope, you’re right! But not all microscope are the same and some are way more powerful than others. Join medical technologist, Stephanie Tucker, to see how much work is needed to be able to see cells on an electron microscope.
Practice Question #4:
Why do samples need to be cut so thin?
Medical School 4 - Mini Med School
Great job, you already have 4 keys!
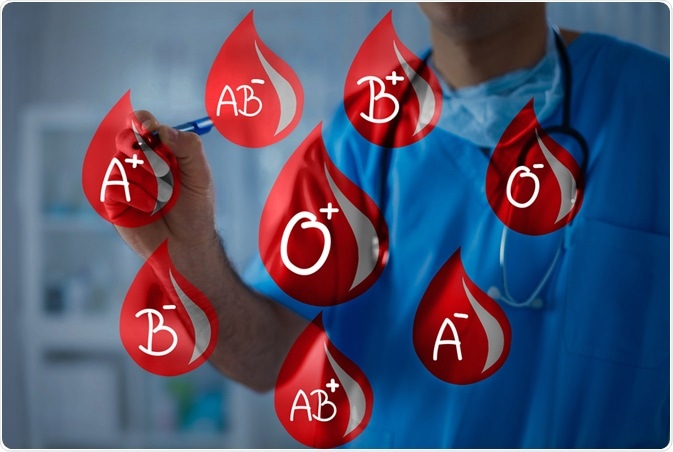
Your fifth key is waiting below with medical technologist Corinne Mercer who explains how medical students are taught and the important differences in human blood – it is not all the same!
Practice Question #5:
What are some reasons you would need to know your blood type? Check all that apply.
Investigating Acids and Bases
Key #6 is right ahead! Great job in the medical school, you whizzed through that!
Next you are on to MUN’s Biochemistry Department with Hannah, Oishi, Jannath, and Jenna for a lesson on acids and bases. If you have every wondered why vinegar tastes sour, keep going to find out! You can do these experiments at home too!
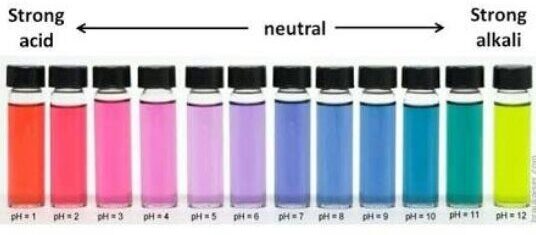
The pH scale from 1-14 indicates how many H+ ions are in a substance. A substance with a lot of H+ ions is acidic and a substance with not very many is basic. Many substances that we use everyday rely on the pH scale, including the foods that we eat. Making cheese starts with milk and acids to produce curds (cheese proteins). You can also use the colour of foods to indicate how acidic or basic they are and how much H+ ions are in them as a result.
Practice Question #6:
On the pH scale what is the number that is considered neutral?
Adventures in the Engineering Department - Activity 1

You’re going to need a big pouch to carry all your keys – you’re not done yet!
The next 3 keys are earned in the Engineering Department and require you to get hands on!
For this activity you will need:
Materials:
- A cup
- A lid made of plastic, cardboard, or cardstock paper
- Water
Directions:
- Before you begin, make sure your lid is flat and is slightly bigger than the opening of the cup
- Gather your materials and set up to do the experiment over a sink or tub
- Pour water into your cup until it is about half full
- Use one hand to hold your cup with water in it and the other to firmly place and hold the lid tightly over the top of your cup. Make sure that the lid is bigger than the top of the cup, as you need to cover the entire top of the cup!
- While holding the lid on top of the cup, turn the cup completely upside down
- Remove your hand from the lid and see what happens!
When you hold a lid on top of the cup and turn the cup upside down, gravity pulls all the water in the cup to the new bottom of the cup. But if gravity pulls the water down, why doesn’t it cause the lid to fall off?
The first reason has to do with physics. The pressure in the cup must match the pressure outside of the cup and forces must be balanced, so there is a force pushing up on the lid to prevent the imbalance a vacuum inside the cup would form.
The second reason is because of the water property called cohesion, which describes what happens to molecules of a substance that really like each other and want to stick together. Water is made up of tons of molecules that really like sticking together, and also like to stick to other, non-oily molecules, like the lid. This property is called adhesion, and water is so cohesive and adhesive that this cool trick works!
Practice Question #7:
What is the water property that describes what happens to molecules of a substance that really like each other and want to stick together?
Adventures in the Engineering Department - Activity 2

Great job, you will solve this mystery in no time!
In order to get key #8, you have to get your thinking cap on for another activity with some materials around your house.
You will need:
- a clear measuring cup or glass
- some water
- a small piece of fruit or vegetable (a small potato, clementine, strawberry or apple pieces would work well)
Instructions:
- Fill your measuring cup half way with water, using the measurements on the cup.
- Drop your fruit or veggie piece in the water
- Check your water level again…has it changed?
- What do you think happened?
Practice Question #8:
What is the term used to describe what happens to the water when the item is added to the cup? Hint: It starts with the 4th letter of the alphabet
Adventures in the Engineering Department - Activity 3

You’re getting close to the end, only a couple more keys to collect. Great job so far on collecting 8 keys!
The last activity in the Engineering Department has you investigating with pepper. Have you ever noticed that pepper floats? Do you know why? Keep going to find out!
You will need the following:
Materials:
- Pepper
- Dish Soap
- Shallow Bowl or Plate
- Water
- Finger or toothpick
Steps:
- Fill your bowl or plate with about an inch of water
- Sprinkle your pepper over the surface of the water, it should float on top
- Before you dip your finger or toothpick in the pepper water, what do you think will happen?
- Dip your finger or toothpick in the dish soap, then carefully place it in the middle of the water
Practice Question #9:
What term is used to describe when something does not like water?
Experiment Relay in the Chemistry Department

You have arrived at the last and final key!! Congrats on a job well-done! One more key to collect and you will have all 10 keys to get to the pets.
Your last stop has you in the Chemistry Department interpreting different kinds of reactions – watch the video for some cool chem in action!
Practice Question #10:
Chemical reactions involve a transfer of energy between the reaction and its surroundings, usually in the form of heat. A reaction that absorbs energy will make its surroundings feel colder, and is called endothermic. A reaction that releases energy will make its surroundings feel warmer, and is called exothermic. Are these reactions in the video all endothermic, or exothermic?
Mystery Solved!!

You have found the animals being hidden in the basement of the new Core Science Building on Memorial University’s campus. The culprit thought this would be a good hiding place because the building is under construction and closed BUT they are no match for your quick thinking and investigative skills! The key that opened the door to the basement was key#6 from the Biochemistry Department. Congrats on solving the mystery and returning the pets to all your neighbours – everyone is very relieved!
Next year, Science Rendezvous will happen on campus (hopefully) in the new Core Science Building above, which is scheduled to open in Fall of 2021. We are very excited to continue Science Rendezvous in the new building – there will be a whale skeleton on display in the whale atrium and lots of exciting things to see. To find out more about the Core Science building go to https://www.mun.ca/csf/.
Practice Question #11:
What is the liquid inside of the cannister?




























































